Our research group focuses on the important electrocatalytic reactions in the field of clean energy conversion, such as ORR, OER, HER, and CO2RR. At present, the reaction mechanism of relevant electrocatalytic reactions is still not clear enough, and the evolution of active centers in the electrocatalytic process also needs to be elucidated. Based on catalyst design, our research group conducts in-depth research around reaction pathways, adsorption states, and evolution of active centers. The main research content includes:
The key issues in the study of electrocatalytic mechanisms
(1)Research on electrocatalytic reaction pathways
Electrocatalytic reactions often involve multiple reaction intermediates and complex proton-coupled electron transfer steps, resulting in sluggish kinetics and insufficient activity. Therefore, it is important to study the pathways of catalytic reactions and lower the energy barrier of the rate-determining step in order to design high-performance catalysts. (1) In order to promote the adsorption of O2 and weaken the binding energy of oxygen intermediates, we reduced the O coverage (ΘO) on the catalyst surface, resulting in the realization of the ORR dissociation path. This facilitated the rapid dissociation of O2 and desorption of oxygen intermediates. (2) We designed Mo-Ce oxide carriers that can promote the in-situ conversion of Co2+-O to Co3+-O in CoSA-MoCeOx@BCT at low voltage. This effectively regulates the lattice oxygen activity of the catalyst, creating a dual-metal-site LOM pathway that breaks the scaling relations and significantly enhances the inherent OER activity. (3) The similar adsorption energies of multiple reaction intermediates in electrochemical CO2 reduction can lead to poor catalyst selectivity and a wide product distribution. Therefore, we initially screened one or more suitable metals as alloy catalysts and then rationally designed catalysts with different alloy ratios or structures, using theoretical calculations to explore the possible reaction paths in electrocatalytic reactions. Representative work: Nat. Catal. 2024, 7, 719-732; Energy Environ. Sci. 2024,17, 3088-3098; Appl. Catal. B: Environ. 2021, 289, 119783.
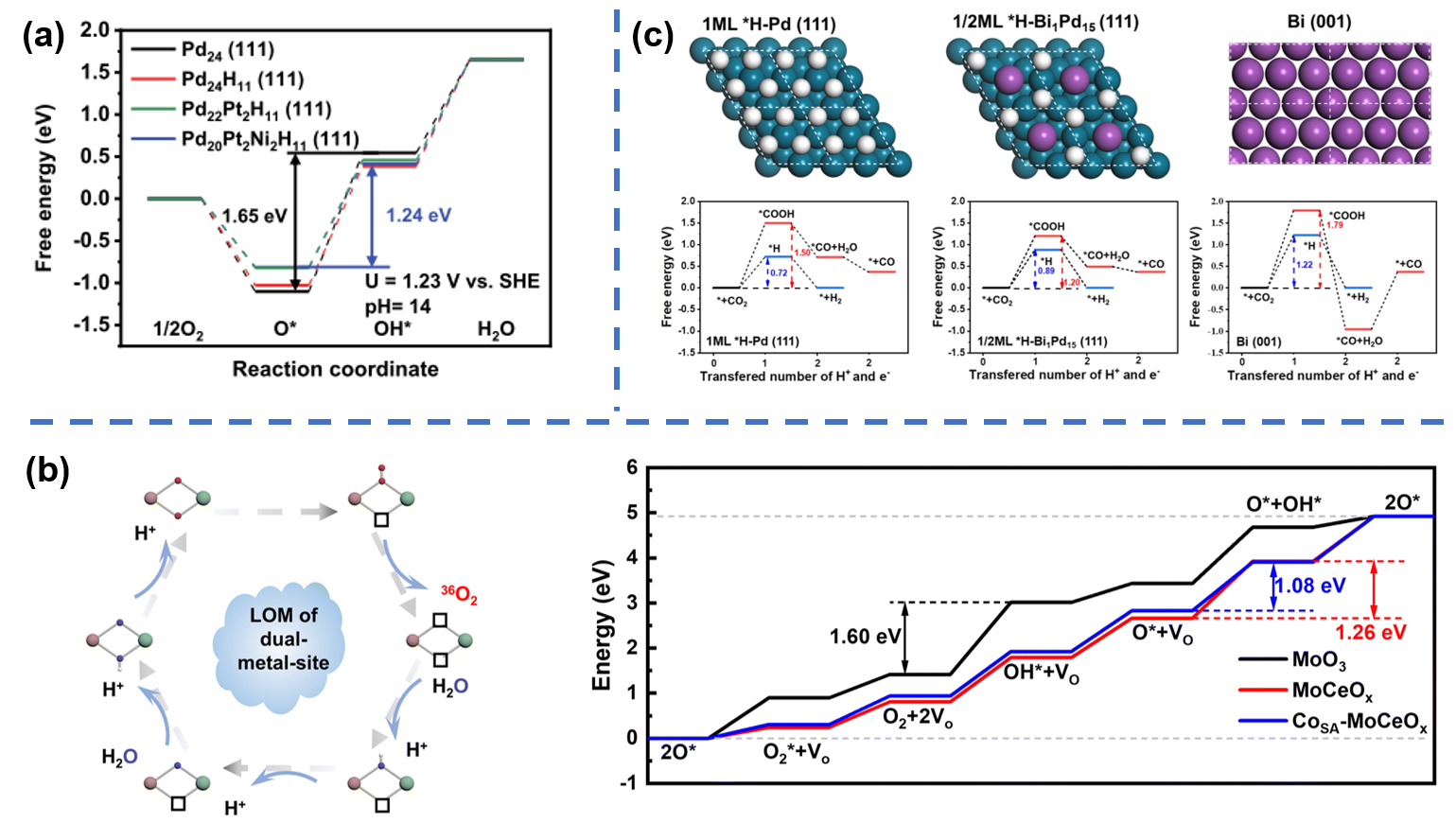
(a)Free energy diagrams for catalysts such as PdPtNiH (111); (b) schematic diagram for the dual-metal-site mechanism of the LOM pathway and free energy diagrams for catalysts such as CoSA-MoCeOx@BCT; (c) geometries and free energy diagrams for CO2RR and HER of catalysts such as Bi1Pd15
(2) Research on catalytic activity based on free energy of adsorption state
During catalytic reaction, the adsorption strength between reaction intermediates and catalysts directly affects catalytic activity and selectivity. According to the Sabatier's principle, too strong adsorption strength is detrimental to the subsequent catalytic reaction, and too weak adsorption strength leads to the inferior catalytic performance. Catalysts with moderate adsorption strength can efficiently facilitate catalytic reaction process. Therefore, the strength of adsorption states of intermediates is an important descriptor of catalytic performance. (1) Investigating the structure-performance relationships of catalysts via strain modulation: We introduced strain effects by doping heterogeneous elements in intermetallic compounds. By plotting volcano plots between activity and strain/stress, we found that catalysts at the top of volcano plots exhibited optimal activity. It is veried that the proper regulation of strain/stress on catalyst surface can optimize the adsorption energy between catalysts and intermediates, achieving efficient design of catalysts. (2) Regulating the adsorption strength via catalytic structures design: Since affinity of O-based adsorbates for Fe is higher than that for Ni, substitution with Fe on the NiOOH surface will enhance the OER activity. Upon doping S into NixFe1-xOOH, surface S residues will significantly reduce the free energy gap for adsorption of O* and OH* on Fe site, thus lowering the OER overpotential of catalysts. Representative work: J. Am. Chem. Soc. 2024, 146, 2033-2042; Angew. Chem. Int. Ed. 2023, 62, e202302134; Adv. Energy. Mater. 2020, 10, 2000179; Adv. Energy. Mater. 2019, 9, 1803771; Angew. Chem. Int. Ed. 2019, 58, 15471-15477; Adv. Mater. 2018, 21, 1800757.
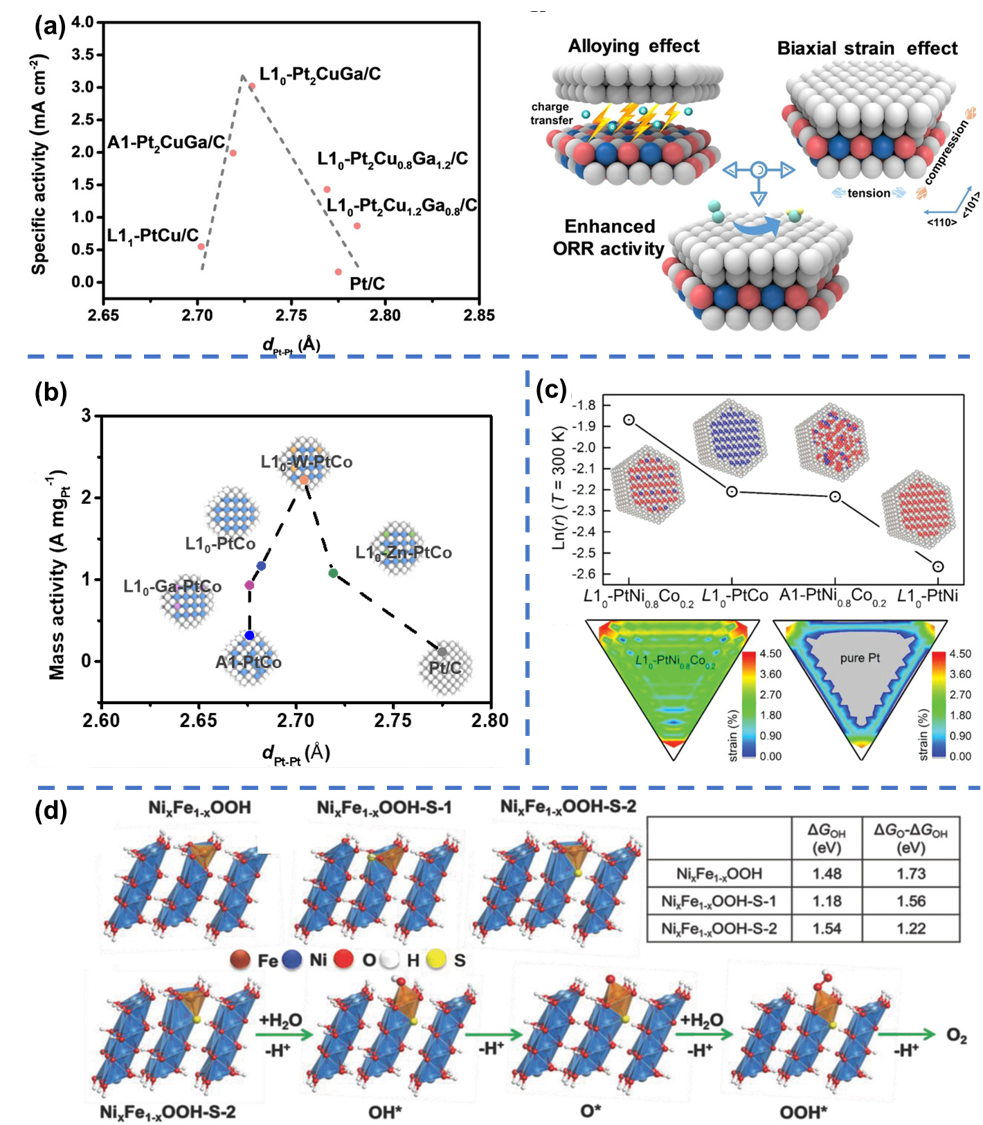
(a) Correlations between ORR SA and dPt−Pt of the L10-Pt2CuGa catalysts; (b) correlations between ORR MA and dPt−Pt of the L10-M-PtCo catalyst; (c) the logarithm of reaction rates on different catalysts and surface strain distributions on L10-PtNi0.8Co0.2 and pure Pt; (d) schematic of the OER reaction mechanism and intermediate structure of NixFe1-xOOH-S
(3)Research on evolution of active centers based on in-situ characterization
Due to variation of potential and special solution environments, the evolution of geometric and electronic structure of catalysts may occur during electrocatalysis, hindering the understanding of actual catalytic sites in catalytic reactions. Therefore, we utilize to observe the evolution of catalysts during electrocatalysis, which helps to gain a deeper understanding in correlations between strutures and performance in electrocatalytic reactions as well as corrosion mechanisms of catalysts. (1) We used in-situ synchrotron radiation to study in-situ structural evolution of ultrathin Pd-based multimetallic nanowires catalysts during ORR, and the results verified that Pd-Pd bond length/strain varies dynamically with applied potential, realizing the promotion of ORR activity. Meanwhile, we verified that the relationships between electronic structures of catalyst and excellent activity from theoretical calculations. (2) We modulated the metal-ligand binding constants to prepare highly stable Fe-N4/C catalysts and unstable Fe-N2~3/C catalysts. Quasi-in-situ EXAFS showed that Fe-N4/C catalyst can maintain its structural stability after stability tests, while Fe-N2~3/C catalysts experienced structural collapse. (3) For in-situ evolution of active sites during OER, in-situ synchrotron radiation and in-situ Raman characterization were used to analyze the valence state and local coordination structure of active sites, so as to derive the correlations between in-situ evolution of valence state, local coordination environments of active sites and activity of OER as well as the reaction pathway during OER. Representative work: Nat. Catal. 2024, 7, 719-732; Energy Environ. Sci. 2024,17, 3088-3098; Adv. Mater. 2021, 33, 2006613.
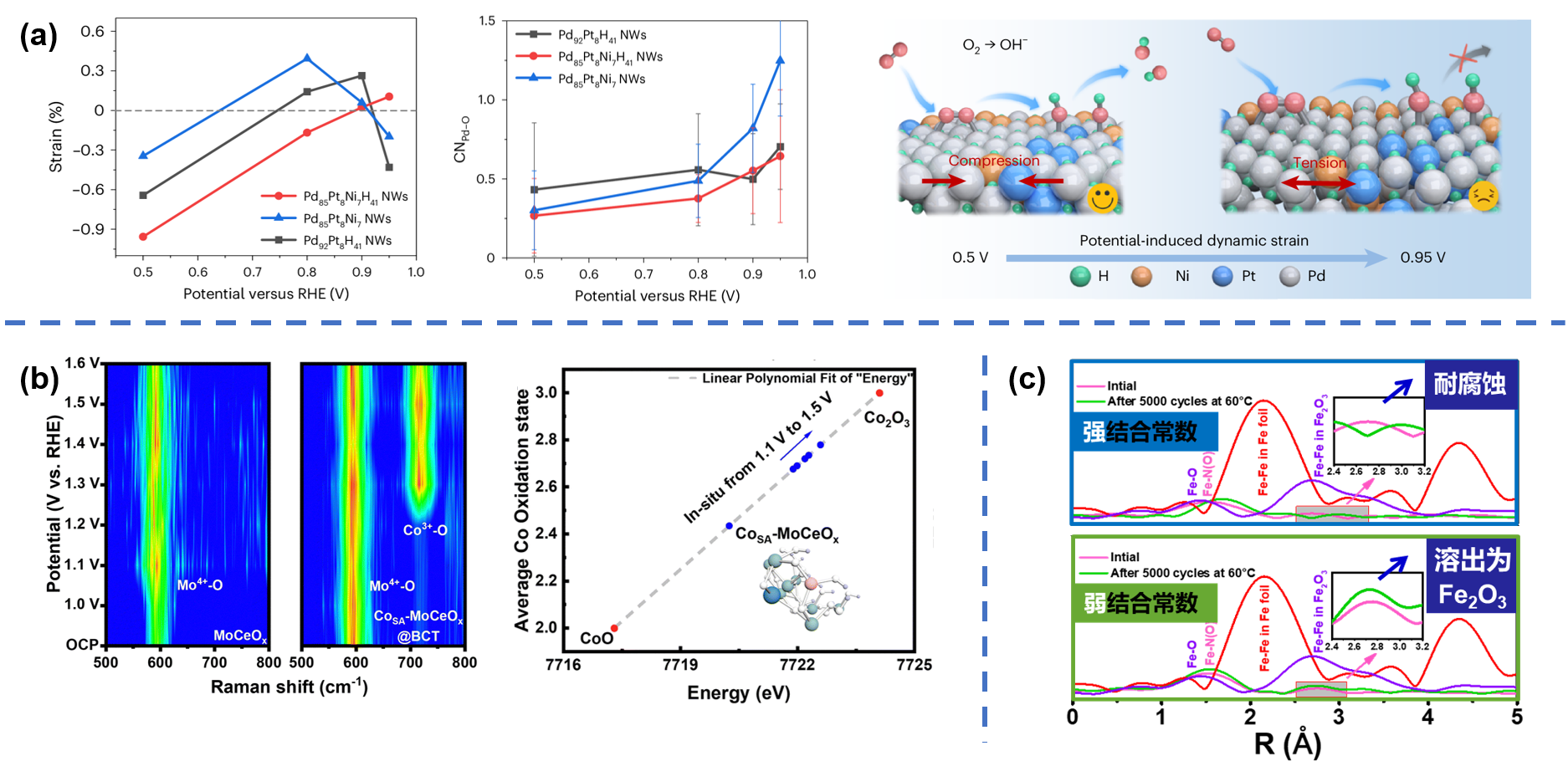
(a)Dynamic change of structural during Operando XAS experiments; (b) dynamic change of structural during in-situ Raman tests; (c) quasi-in-situ EXAFS characterization of the structural changes of M-N-C materials before and after stability tests
