The electrochemical reactions (ORR, HOR, OER, HER) involved in hydrogen fuel cells, SPE water electrolysis and other types conversion devices all have the problem of sluggish kinetics, at present, a large amount of precious metals such as Pt and Ir are still needed as catalysts to accelerate the reaction. Therefore, the design and synthesis of new, efficient, low-cost, long-life electrocatalysts has important scientific research value and practical significance for the development and utilization of new energy sources. Related catalysts have a series of problems such as high cost and insufficient activity/stability. In this regard, our research group precisely controls the catalyst structure at the atomic level to develop high-performance nanocatalysts.
The main research of our group are as follows:
(1)Design of new structure catalysts based on crystal phase engineering control
The crystal phase structure and atomic arrangement of catalysts are closely related to their catalytic activity and structural stability. These structures affect the surface Pt atom d-band density of states curve and d-band center, which in turn changes the binding energy between Pt and oxygen-containing intermediates, affecting catalytic activity. Moreover, the atomic packing of the catalyst significantly influences its corrosion resistance in harsh environments. The research focus of our group includes regulating the phase structure of nano-catalysts through novel and reasonable synthesis methods and treatment approaches to enhance the activity and stability of catalysts in new energy devices and deeply understand the structure-activity relationships. For instance, by constructing oxygen vacancies, traditional disordered A1-PtM alloy catalysts can be transformed into new ordered L10-PtM catalysts. Completely ordered L10-PtFe nanocrystals, for example, show no significant changes in electrochemical performance and catalyst composition after 20,000 cycles. Introducing Cr elements into ultrafine ordered L10-PtFe nanocrystals effectively acts as an electronic buffer, stabilizing the catalyst structure and preventing Pt/Fe dissolution by reducing valence states and weakening tensile strain. In H2-air PEMFC operation, the L10-Cr-PtFe/C cathode exhibits an extremely high initial mass activity (MA) of 1.41 and 1.02 A mg Pt-1 (at 0.90 V), and beginning-of-life (BOL) rated power of 14.0 and 9.2 W mg Pt-1, with total cathode Pt loadings of 0.075 and 0.125 mg Pt cm-2 (anode loading of 0.025 mg Pt cm-2). After 60,000 ADT cycles at 0.8 A cm-2, the potential loss is only 20 mV, exceeding the US Department of Energy's 2025 technical targets. Using a strategy of bond weakening induced by low-melting-point elements (Ga, Sn, In), we effectively reduced the phase transition temperature from disordered to ordered structures (<500℃). This allowed us to prepare high-Pt-content (≥40wt%) L10-Pt-M-M' intermetallic compound nanocrystals and achieve a laboratory-scale production of 10 grams, promising large-scale production of ultra-fine ordered nanocrystals. The high-loading ordered L10-PtNiGa catalyst prepared based on this strategy exhibited excellent activity and stability in heavy-duty fuel cell tests, with an initial current density of 1.68 A cm-2 at 0.7 V, which is 50% and 28% higher than commercial Pt/C and PtNi/C, respectively. After 90,000 cycles, the current at 0.7 V remained at 1.33 A cm-2. Both the activity and retention rate exceed the indicators set by the Ministry of Science and Technology's 14th Five-Year Plan for Catalysis and the U.S. Department of Energy, making it the most stable heavy-duty fuel cell catalyst under current working conditions. (Representative works include: Nat. Mater. 2024, 23, 1259-1267; J. Am. Chem. Soc. 2024, 146, 3; Adv. Energy Mater. 2019, 9, 1803771; Joule 2019, 3, 956-991; J. Am. Chem. Soc. 2015, 137, 7071; Nano Lett. 2015, 15, 2468)
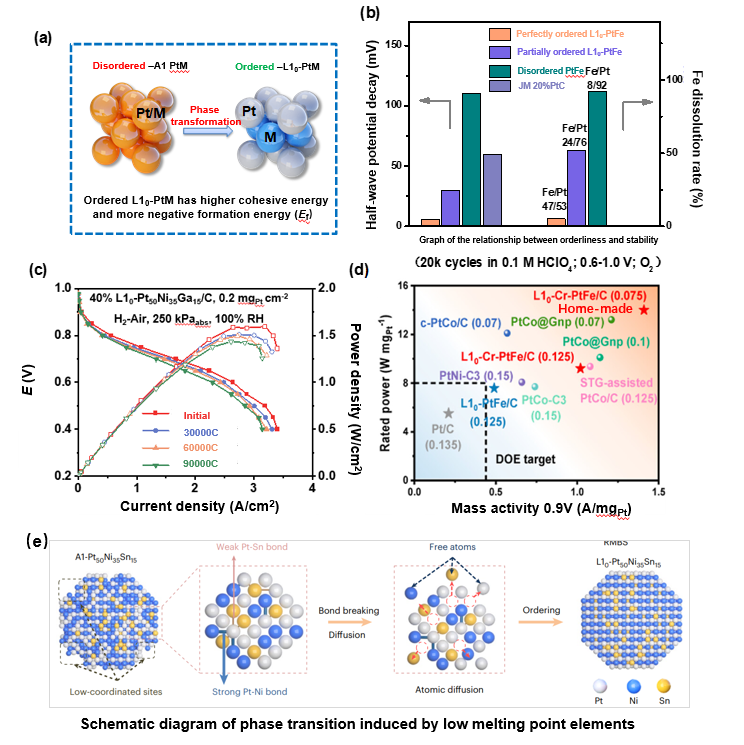
(a) Schematic structural diagram of ordered L10-PtM and disordered A10-PtM. (b) The relationship between the degree of order and the catalytic stability and composition stability of L10-PtM. (c) H2–air fuel cell polarization curves. (d) Comparison of the mass activity and rated power for catalysts from our catalyst and previous works.(e)Diagram of Phase Transition Induced by Low-Melting-Point Elements.
(2) Design of new catalysts based on physical and chemical parameter control
The thermal/kinetic physical and chemical parameters of the catalyst, such as strain, cohesive energy, and surface energy, have a crucial impact on its catalytic activity and cycle stability. Thus, our research group focuses on the physical and chemical parameters of each element and alloy system, regulates them from multiple perspectives of thermodynamics (structure formation energy, surface energy, etc.) and kinetics (vacancy formation energy, phase change activation energy, etc.) to optimize the performance of the catalyst both in half cell and device. We increased the binding energy/cohesive energy of the non-noble metal M-N-C catalyst, by regulating the catalyst binding constant/force, the increased cohesive binding energy effectively inhibiting the dissolution and agglomeration of active centers during the catalytic reaction process, and achieving long-term stability of the catalyst in half-cells and PEMFCs. For example, we developed an effective and universal strategy to enhance the stability of the non-noble-metal MNx/C catalyst in proton exchange membrane fuel cells (PEMFCs) by improving the bonding strength between metal ions and chelating polymers, i.e., poly(acrylic acid) (PAA) homopolymer and poly(acrylic acid–maleic acid) (P(AA-MA)) copolymer with different AA/MA ratios. The optimized P(AA-MA)–Fe–N catalyst exhibits extraordinarily high activity and stability both in half-cell and H2–air fuel cells, with an E1/2 loss of only 6 mV after AST 60 °C and a nearly unchanged current density for 37 h at 0.55 V, respec-tively, among the best overall performance in its class reported so far. (Representative papers: Angew. Chem. Int. Ed. 2019, 58, 15471; Adv. Energy Mater. 2020, 10, 2000179; Adv. Mater. 2021, 33, 2006613)
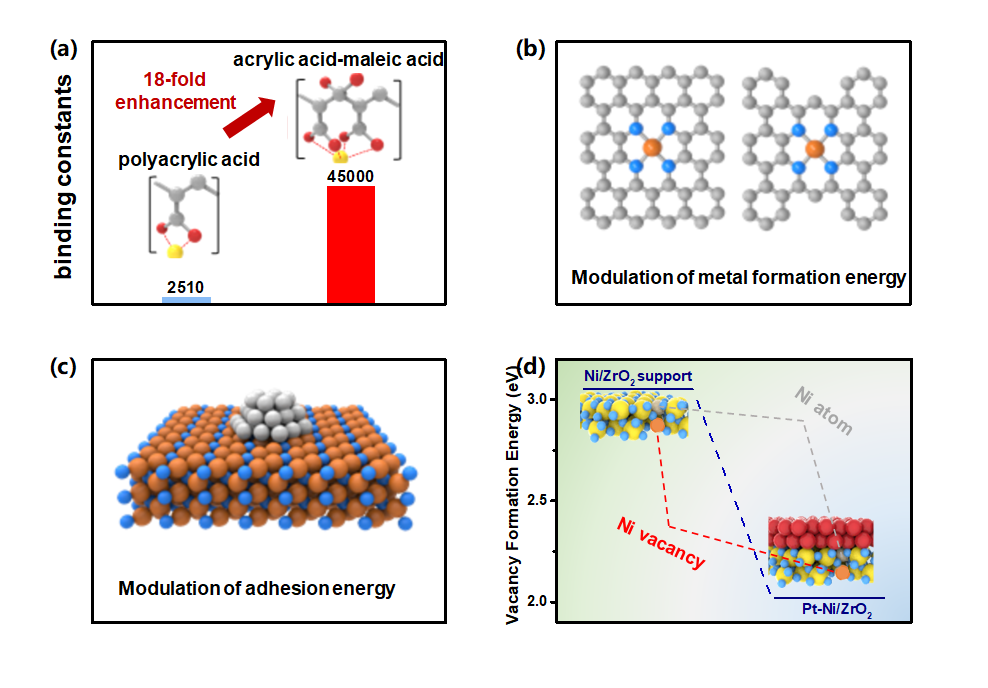
(a) Comparison of binding constants between Fe and acrylic acid homopolymer or (acrylic acid-maleic acid) copolymer system. (b) Comparison chart of fuel cell performance of strong binding constant P(AA-MA)–Fe–N catalyst with literature. (c) Schematic diagram of M-N-C structure. (d) Schematic diagram of supported metal catalyst.
(3)Research on large-scale preparation technology of catalysts
The commercialization of catalysts requires not only a rational structural design to enhance their activity and stability but also the development of technological routes for large-scale production. Therefore, another important research direction of our group is the development of efficient and green synthesis technologies to promote the large-scale production of catalysts, bridging the gap between laboratory preparation and industrial production. At present, relevant technologies are in the stage of transformation of results: 1) By introducing low-melting-point elements, the activation energy of nanocrystal phase transition is regulated, and the phase transition temperature of nanocrystals is significantly reduced (less than 500 °C), laying the foundation for the large-scale preparation of ultrafine ordered nanocrystals; 2) Developing green and environmentally friendly non-organic synthesis methods to achieve kilogram-level preparation of high-loading (60%) Pt/C and Pd/C catalysts. We also develop green and environmentally friendly non-organic synthesis methods to achieve kilogram-scale production of platinum-carbon and palladium-carbon catalysts. We have cooperated with Wuhan Zhongyu Power System Co., Ltd. and other companies to conduct relevant application tests on ordered alloy catalysts in hydrogen fuel cell stack products. We have cooperated with Zhongshun Environmental Protection Co., Ltd. on recycling waste precious metals as raw materials and preparing high-efficiency catalysts on a large scale. At present, more than 10 invention patents have been authorized for related technologies. (Representative works include: Nat. Mater. 2024, 23, 1259-1267; Adv. Mater. 2022, 34, accepted; ACS Nano 2020, 14, 10115;Chinese J. Catal. 2020, 41, 847.)

Schematic Diagram of Catalyst Mass Production Design and Pilot Plant,catalyst pilot product, fuel cell stack diagram,
Collaborating Party and Third-Party Testing Report for the Product
