Based on reaction mechanism research and catalyst design, our research group further develops electrochemical energy conversion and storage devices and systems such as hydrogen fuel cells, electrolysis of water, and new secondary batteries, in order to achieve real industrial applications. The main research contents include:
(1)Hydrogen fuel cell devices
Proton Exchange Membrane Fuel Cell (PEMFC) is a new type of energy device that directly converts chemical energy into electrical energy. It has the advantages of high energy conversion efficiency, low operating temperature, clean and green. But there are a series of problems such as slow cathode kinetics, high cost of catalyst and poor stability. Based on the developed high-performance low-platinum nanocrystalline catalysts and transition metal-nitrogen-carbon (M-N-C) non-precious metal catalysts, our research group has carried out the integration of PEMFC devices and realized the long-term stable operation of the catalysts in the devices. At present, the performance of the L10-W-PtCo catalyst developed by our research group in PEMFC devices has exceeded the relevant hydrogen energy project targets of the Ministry of Science and Technology and the US Department of Energy's 2020 targets. The stability of this catalyst is at the highest level reported in the literature at that time. The L10-Ga-PtNi catalyst developed by the group has maintained more than 80% of its activity under the test conditions of 90,000 cycles when applied to heavy duty vehicles (HDVs), which is at the top level of the industry. Representative publications:Nat. Catal. 2024, 7, 719-732; Nat. Mater. 2024, 23, 1259-1267; Angew. Chem. Int. Ed. 2024, 63, e202400751; Adv. Mater. 2021, 2006613; Adv. Energy Mater. 2020, 10, 2000179; Small 2021, 17, 2100735.
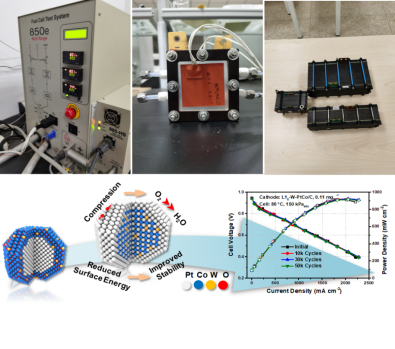
Fuel cell test system and catalyst performance response
(2)Water splitting and CO2 electroreduction devices
The production of hydrogen by water electrolysis represents a sustainable and efficient method for the generation of high-purity hydrogen, a substance that is essential for the advancement of future renewable energy technologies. This is achieved by the realization of the water-hydrogen-oxygen cycle, which is employed to produce hydrogen fuel. Currently, water electrolysis devices exhibit suboptimal reaction kinetics and high overpotentials. Furthermore, the electrocatalysts are subjected to strongly acidic or alkaline environments during the reaction process, which causes corrosion problems and hinders further applications. Our group has designed corrosion-resistant low-loading noble metal as well as non-precious metal catalysts for alkaline water electrolysis, PEM water electrolyzers, and seawater electrolyzer. These devices have been integrated to achieve highly efficient hydrogen production from electrolytic water. Representative works: Energy Environ. Sci. 2024, 17, 3088; Nat. Commun. 2023, 14, 3934; ACS Catal. 2023, 8, 5194; Appl. Catal. B: Environ. 2022, 302, 120862.
The CO2 liquid-phase electrolyzers are the most common mobile phase electrolyzers. It has been demonstrated to successfully address the limitations of the traditional H-type electrolyzers, including the dissolution and diffusion of CO2. The electrolyzer has the capacity to enhance the reaction currents by one to two orders of magnitude, and it can be used with high-concentration alkaline electrolyte (e.g., KOH), which improves the reaction selectivity. Based on the developed CO2 reduction catalysts with high selectivity and long-term stability, our group has carried out the integrated design of devices such as CO2 liquid-phase flow electrolyzers to realize the efficient reduction of CO2 to C1/C2 products. Representative works: ACS Catal. 2024, 14, 489; Chinese J. Catal. 2022, 43, 1680; Appl. Catal. B: Environ. 2021, 289, 119783.
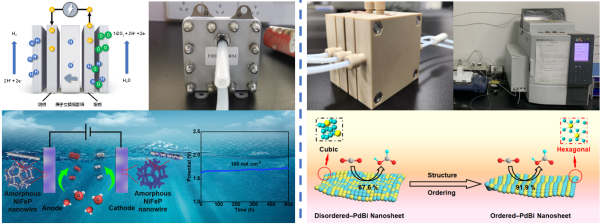
Electrolytic water hydrogen production and CO2 electric reduction devices
(3)New Secondary Batteries
Renewable energy such as solar energy, wind energy, and hydraulic energy have been gradually applied. However, due to the instability of their power output, it is difficult to directly connect these clean energy sources to the grid, and large-scale energy storage equipment needs to be developed to support them. Our research group has carried out a series of work on rechargeable batteries with high energy density and long cycle life, mainly focusing on 1) the application of nanocatalysts in the catalytic conversion of polysulfide ions in the cathode of lithium-sulfur batteries; 2) the optimization of electrode materials for lithium-ion batteries; 3) High-performance rechargeable zinc-air battery design. Representative publications: Adv. Mater. 2018, 30, 1800757; ACS Nano. 2020, 14, 10115;Nanoscale. 2020, 12, 584; Nanoscale. 2018, 10, 5634; Electrochimica Acta. 2018, 282, 973-980.
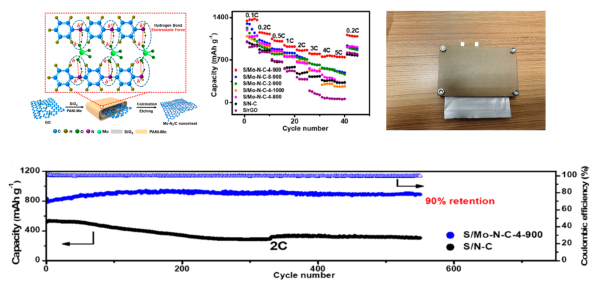
Lithium sulfur battery cathode material design and soft pack battery performance display
